
Technology Platform
An end-to-end integrated platform designed for better surgical results, reduced revisions, and improved long-term outcomes.
Advancing what’s possible through personalized technology solutions.
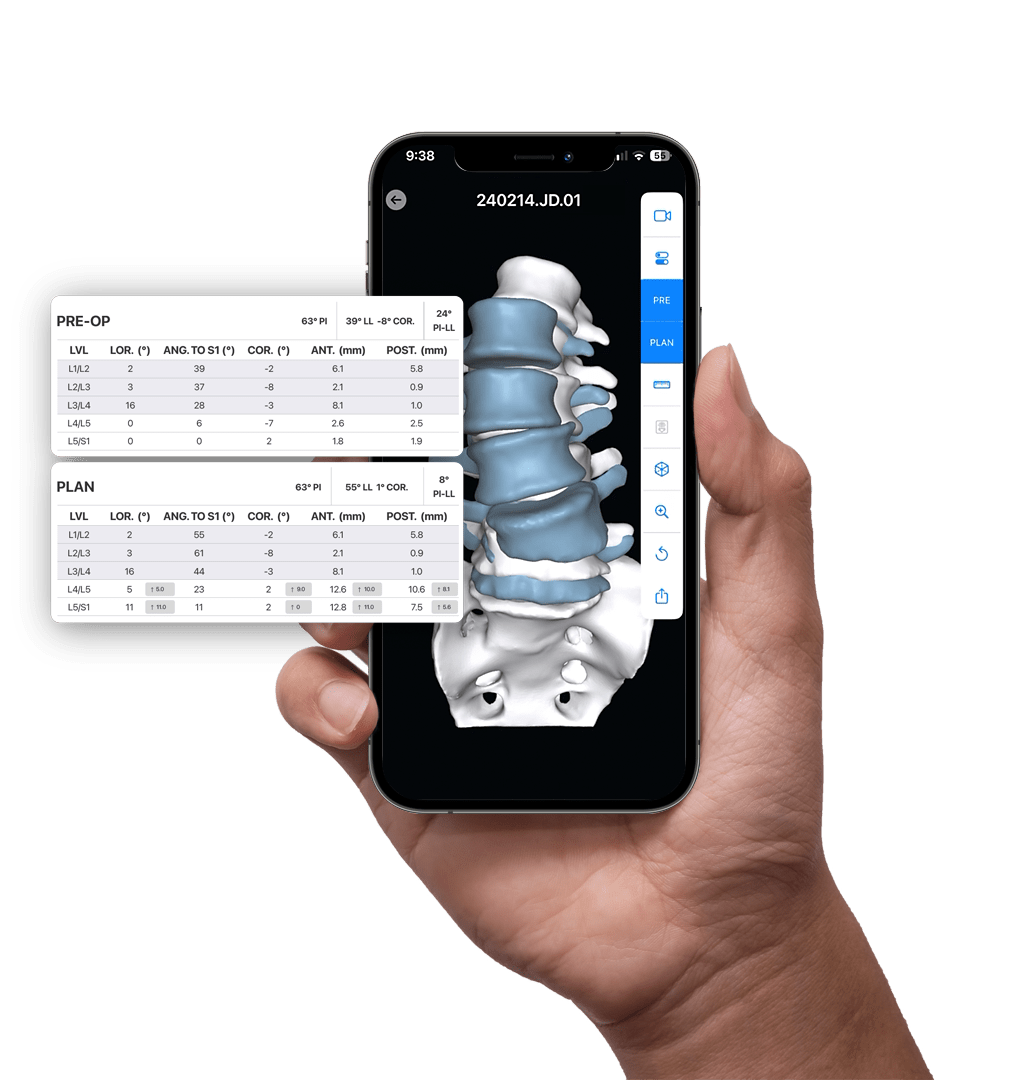

PRE-OP
AI-enabled 3D Surgical Planning
The aprevo® planning software combines Al-enabled segmentation and prior outcomes data to develop personalized surgical plans and devices for each patient.
Detailed 3D patient data, the latest spinopelvic parameter classifications, normative range targets, and real-world post-operative data are leveraged to inform and enhance each patient’s surgical plan.
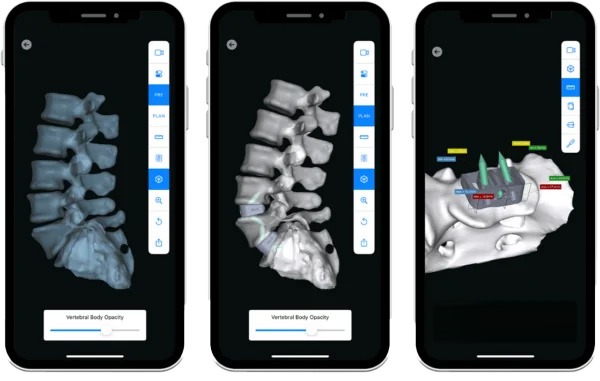
INTRA-OP
Advanced Visualization & Precision Execution
3D correction and precision alignment are built into each aprevo® personalized interbody device helping to ensure that each patient’s personalized treatment extends seamlessly into the operating room.
The myaprevo® app provides each surgeon and the OR staff with an intuitive and detailed 3D plan and device visualizations that aid in the ability to attain precise correction and predictably achieve the surgical plan.
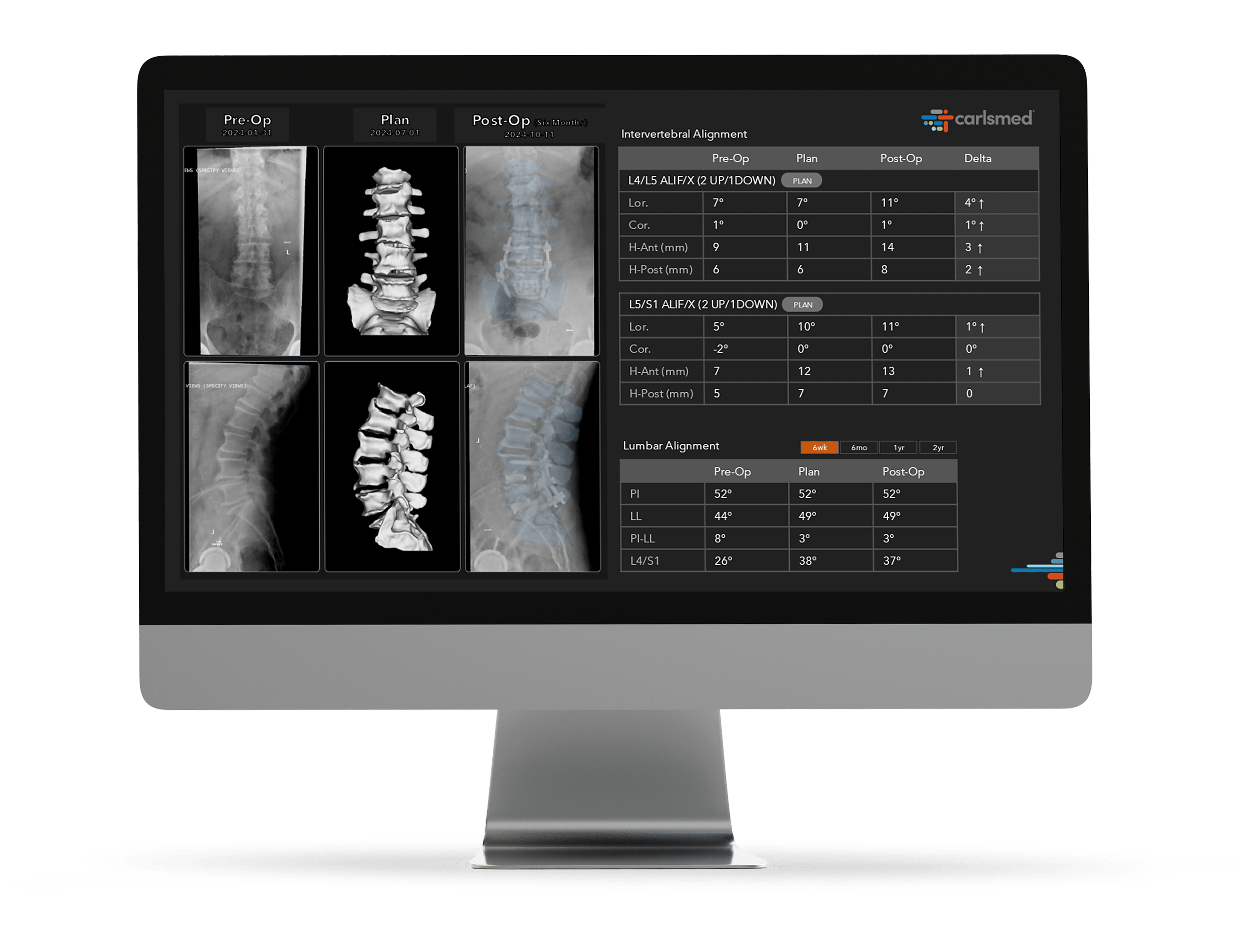
POST-OP
Enhanced Reproducibility and Integrated Intelligence
Personalized digital insights are translated into action to help improve care and enhance reproducibility. aprevo® intelligence leverages AI-driven systems to analyze post-operative surgical data to help surgeons achieve greater precision and predictability.

CT Scan
Each patient’s CT scan is used to create a precise 3D model of their spine, providing a detailed foundation for personalized surgical planning.
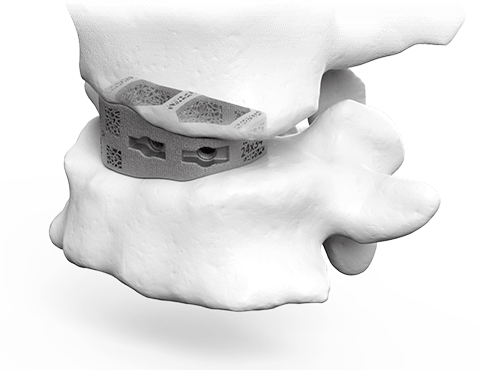
3D Plan & Design
aprevo® AI-enabled software is used to create surgical plans that precisely match the surgeon’s desired alignment and correction. Based on the plan, patient-specific devices are designed to fit the patient’s unique anatomy.

Manufacture
The patient’s aprevo® devices are 3D printed after the surgical plan and device designs are approved by the surgeon.
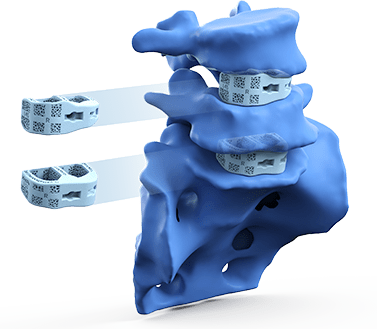
Device Delivery
The patient’s personalized devices are delivered to the operating room sterile and ready for implantation during surgery.
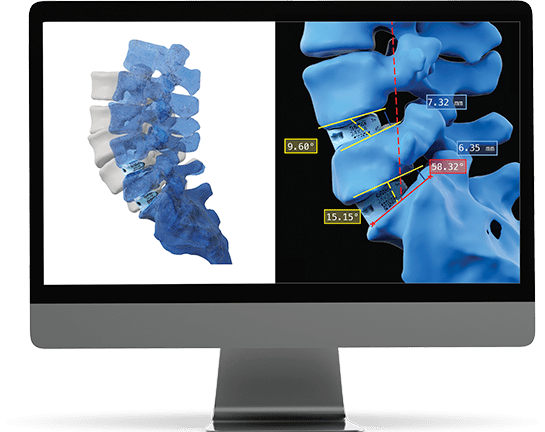
Data Intelligence
The patient’s post-operative images are measured and analyzed to confirm that the operative goals for each patient were achieved.

